Parler
Parler Gab
Gab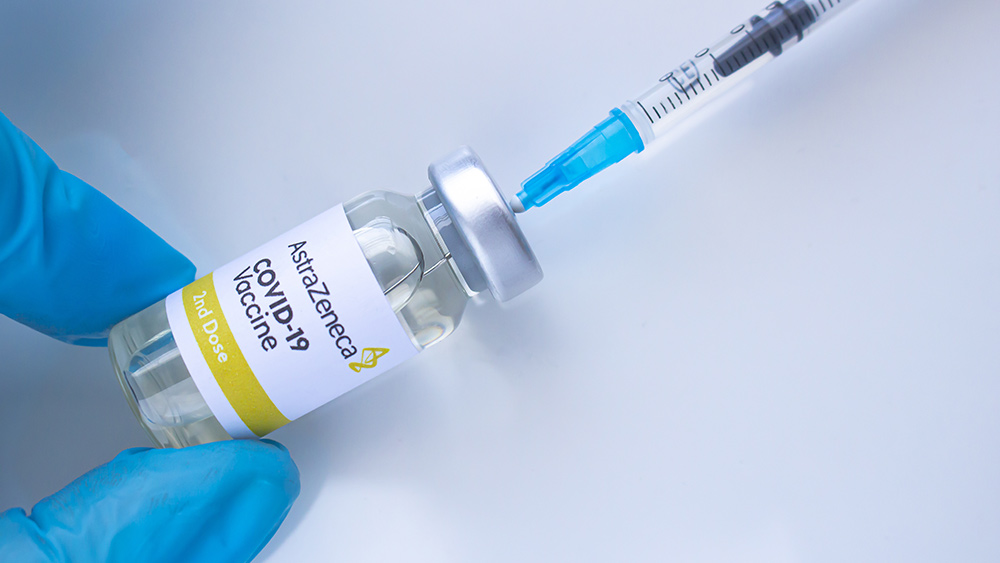
Norway follows Denmark in banning AstraZeneca
Norway appeared to follow its Scandinavian neighbor Denmark in permanently suspending the use of the AstraZeneca vaccine. Danish health authorities imposed a total ban on the vaccine on April 15. Danish Health Authority (SST) Director-General Søren Brostrøm said in a statement: "Overall, we must say that … there is a real and serious side effect signal in the vaccine from AstraZeneca. Based on an overall consideration, we have therefore chosen to continue the vaccination program for all target groups without this vaccine." (Related: Denmark permanently bans Covid-19 vaccine from AstraZeneca over deadly blood clots.) However, Brostrøm clarified that the decision to drop the vaccine should solely be seen in a Danish context. "I understand very well why other countries will use it," he remarked. Copenhagen first suspended the use of AstraZeneca's COVID-19 vaccine in March after two people suffered from severe blood clots following inoculation with the shot. According to the SST director, joint studies based on Danish and Norwegian health data estimate that one in 40,000 people immunized with the AstraZeneca vaccine is at risk for this side effect – regardless of age and gender. Brostrøm continued: "In the midst of an epidemic, it has been a really difficult decision to continue our vaccination program without an effective and readily available vaccine against COVID-19. However, we have other vaccines at our disposal." Nevertheless, health authorities have insisted the AstraZeneca COVID-19 vaccine is safe to use. (Related: 20+ countries suspend use of AstraZeneca vaccine, but regulators insist 'benefits outweigh risks'.) European Medicines Agency (EMA) Director Emer Cooke previously defended the AstraZeneca vaccine, saying that "there is no indication that vaccination has caused these [serious] conditions." She added: "A situation like this is not unexpected when you vaccinate millions of people." World Health Organization (WHO) officials also shared the EMA director's sentiments. During a March 15 press conference, WHO Director-General Tedros Adhanom Ghebreyesus said the global health body is reviewing available safety data. He added that the adverse reactions linked to AstraZeneca's vaccine "shows that the [vaccine] surveillance system works and effective controls are in place. WHO Chief Scientist Soumya Swaminathan remarked in the same press conference that it is best for countries to continue using the AstraZeneca vaccine. Visit VaccineDamage.news to read more about the risks linked to the AstraZeneca COVID-19 vaccine. Sources include: TheEpochTimes.com ChildrensHealthDefense.org BusinessToday.in SST.dk MSN.com NDTV.comDeborah Birx hid covid info from Trump, altered CDC guidelines without approval
By Ethan Huff // Share
Germany’s birth rate improbably falls by 11% in the first quarter of 2022
By Lance D Johnson // Share
By Mary Villareal // Share
Common signs and symptoms of magnesium deficiency
By Olivia Cook // Share
Tulsi Gabbard takes aim at censorship: Justice for the 'Disinformation Dozen'
By newseditors // Share
Trump accused of ‘Deceptive’ tariff calculations – Asian economies deny claims of high import taxes
By finnheartley // Share