Parler
Parler Gab
Gab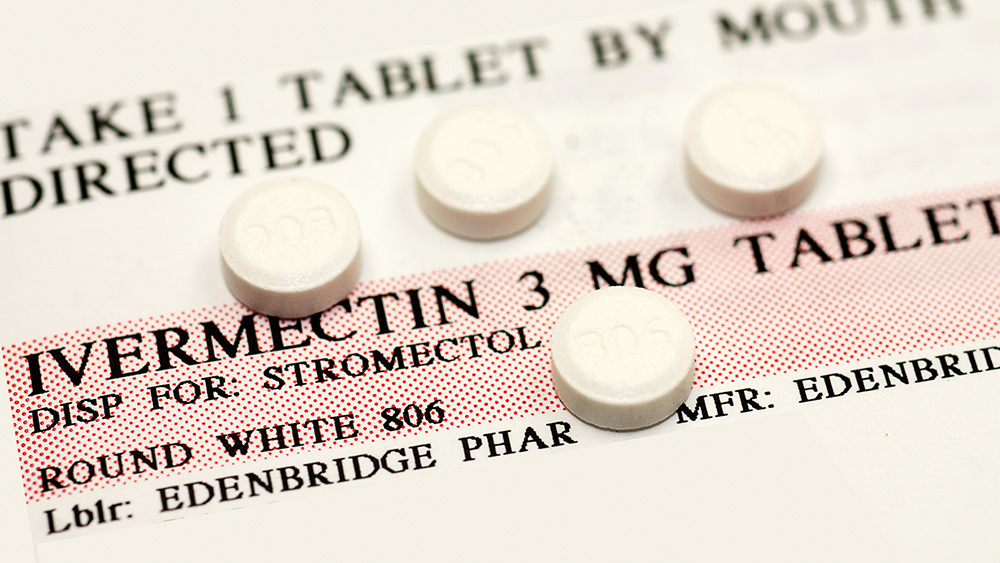
The FDA is an enemy of We the People
It has been about two weeks since Judge Willett's ruling, and the social media post in question, as well as others, is still up on X unchanged. "You are not a horse," reads the Aug. 21, 2021, X post that got the FDA in trouble in the first place. "You are not a cow. Seriously, y'all. Stop it." The webpage that the FDA linked to in this particular social media post also remains unchanged, ordering Americans that they "should not use ivermectin to treat or prevent COVID-19." To be fair, the appeals court never actually told the FDA to modify or take down the posts. The case has also been remanded to a lower court for consideration on its standing. Still, Dr. Robert Apter, the lead plaintiff in the case that ultimately led to Willett's ruling, says the FDA has an obligation to take down these offending posts that are still up in violation of the law. "From an ethical point of view, the FDA has been told not to do what they are doing," Dr. Apter is quoted as saying. "They have an ethical and moral obligation to follow the court's directive and stop giving advice against using effective repurposed drugs for early treatment of COVID." When asked by at least one independent media outlet for comment on the matter, the FDA reportedly declined. The FDA did, however, issue a statement following Judge Willett's ruling. It states that ivermectin is approved by the agency for other uses, but not for COVID, even though Big Pharma companies have been repurposing FDA-approved drugs for decades without consequence. The FDA stipulated that it "has not authorized or approved ivermectin for use in preventing or treating COVID-19, nor has the agency stated that it is safe or effective for that use." "Health care professionals generally may choose to prescribe an approved human drug for an unapproved use when they judge that the unapproved use is medically appropriate for an individual patient," the agency added. This process is known as off-label prescription, and it is common in the United States as drug companies look for new ways to keep the profits flowing once a given drug loses its luster for its officially approved purpose. "The decision is pretty clear that the FDA is not a physician, and that while it might have authority to inform the public, it can't endorse particular treatments or advise on how to approach any specific illness," commented Jared Kelson, an attorney representing the doctor plaintiffs in the case. The latest news about the corrupt and criminally run FDA can be found at FDA.news. Sources for this article include: TheEpochTimes.com NaturalNews.comForever chemicals pose greater cancer risk to women than men, study finds
By Zoey Sky // Share
Stevia kills Lyme disease pathogen better than antibiotics (preclinical study)
By News Editors // Share
Leader in Richmond Democrat Party group posted bomb threat against Andy Ngo Virginia talk
By News Editors // Share
Mouthwashes contain deadly chemicals linked to HEART problems, CANCER, study finds
By Ethan Huff // Share
Governments continue to obscure COVID-19 vaccine data amid rising concerns over excess deaths
By patricklewis // Share
Tech giant Microsoft backs EXTINCTION with its support of carbon capture programs
By ramontomeydw // Share
Germany to resume arms exports to Israel despite repeated ceasefire violations
By isabelle // Share