Parler
Parler Gab
Gab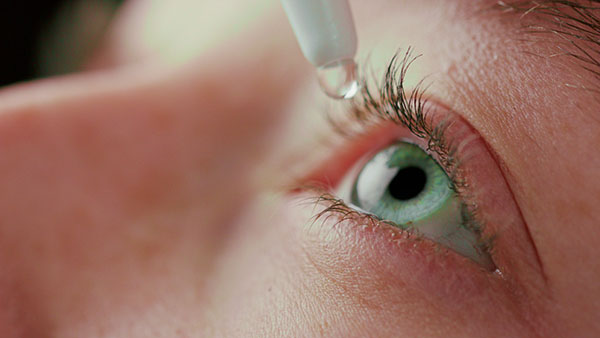
Contaminated eye drops linked to fatal outbreak of drug-resistant bacteria
In April, CBS News reported that federal inspection records from Feb. 20 through March 2 showed dozens of issues at Global Pharma Healthcare Pvt. Ltd.’s manufacturing plant in India – ranging from dirty equipment and clothing to missing safeguards and procedures. The investigation was prompted by a swelling multistate outbreak of a rare and extensively drug-resistant bacteria – Pseudomonas aeruginosa – that was linked to eye drops sold under the brand names EzriCare Artificial Tears and Delsam Pharma’s Artificial Tears and Artificial Ointment. On March 22, the Centers for Disease Control and Prevention (CDC) update reported that out of 81 patients identified across 18 states with the bacteria, 14 people have lost their vision; four have had their eyeballs removed; and the death toll has climbed to three. (Related: Three people died after using eye drops contaminated with rare bacterial superbug.) Confirmed infection cases were from California, Colorado, Connecticut, Delaware, Florida, Illinois, North Carolina, Nevada, New Jersey. New Mexico, New York, Ohio, Pennsylvania, South Dakota, Texas, Utah, Washington and Wisconsin. Patients reported experiencing symptoms, which included blurry vision; clear, green, or yellow discharge from the eyes; eye discomfort or pain; feeling of something in the eye (foreign body sensation); increased sensitivity to light; and redness of the eye or eyelid. The federal investigators' report included the following:- Evidence of poor cleaning procedures throughout the facility – investigators reported surfaces that come into contact with the packaging were "not cleaned, decontaminated, sanitized or sterilized" at all
- A product-filling room that had "soft, unsmooth and cracked sealant, protruding nails and nail holes across its walls"
- Discolored and worn-out booties being used in the company’s "clean rooms"
- Absence of a tracker or monitoring record or have studies that show "how many times plant-issued clothing could be reused by workers"
- Substantial gaps in written procedures and training for workers at the plant
- Unexplained discrepancies wherein an inspector "saw a black-brown greasy deposit" on a part of one of the machines used to fill its products into appropriate receptacles, yet the logs claimed that particular machine had been cleaned just weeks before and had not been used since
- Falling short of double-checking the ingredients it used to produce its products by merely relying on assurances from its suppliers
- Not using appropriate preservatives in its reusable eye drop bottles raise the risk of bacteria growing in already opened bottles
More related stories:
Over 2M at-home COVID-19 test kits recalled due to false positive results. FDA announces voluntary recall of contaminated eye drops that could blind people. FDA issues warning on several over-the-counter EYE DROPS that could cause infection and partial vision loss. Sources include: DailyMail.co.uk CBSNews.com 1 FDA.gov 1 USAToday.com CDC.gov CBSNews.com 2 FDA.gov 2 CBSNews.com 3 OptometryTimes.com Brighteon.comThe life-saving properties of garlic revealed
By News Editors // Share
U.K. approves CRISPR gene editing to create GMO humans
By Ethan Huff // Share
Congresswoman MTG vows to uncover truth behind COVID-19 injections
By Richard Brown // Share
Ukrainian commander laments INACCURACY of U.S.-provided M109 Paladin howitzer
By Ramon Tomey // Share
Governments continue to obscure COVID-19 vaccine data amid rising concerns over excess deaths
By patricklewis // Share
Tech giant Microsoft backs EXTINCTION with its support of carbon capture programs
By ramontomeydw // Share
Germany to resume arms exports to Israel despite repeated ceasefire violations
By isabelle // Share