Parler
Parler Gab
Gab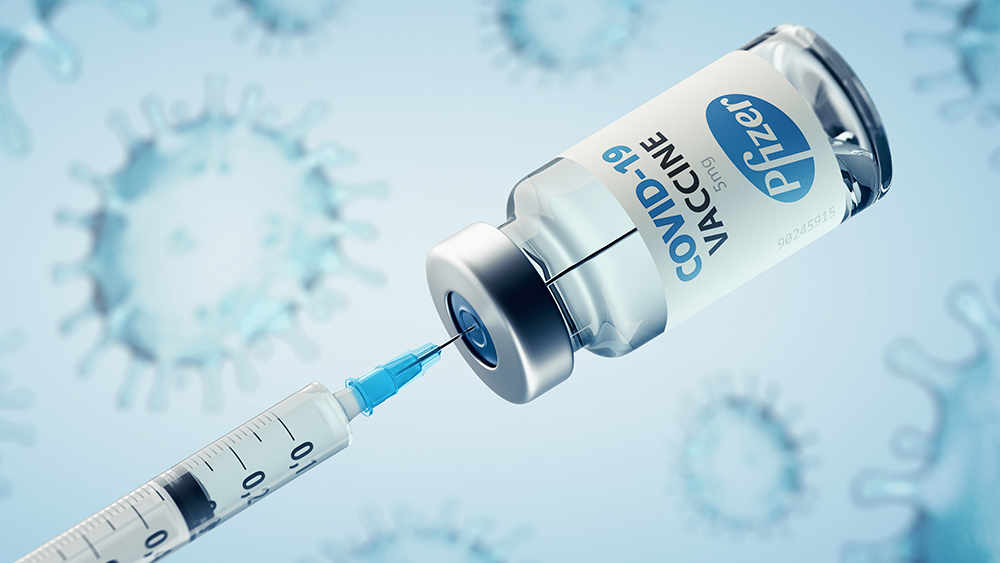
Pfizer’s FDA-approved COVID vaccine nowhere to be found
The Biden administration and other states heralded the approval of Pfizer’s comirnaty as a landmark moment after getting FDA approval on August 23. But here’s the catch: Comirnaty is nowhere to found. A separate product by Pfizer, which was given emergency use authorization, is the one available in the market. Pfizer explained that comirnaty isn’t available yet because of the presence of the EUA shots, but assured the public that it will soon be available. Questions were also raised on why the FDA won’t approve the available product despite claims that it is the same as comirnaty. Observers noted that Pfizer wants comirnaty to become available for all ages in order to get an additional layer of protection from legal liabilities. Steve Kirsch on his Substack said that comirnaty will magically appear in the market if approved for kids. “The reason comirnaty isn’t available is because those shots would expose the company to liability since the fully licensed product doesn’t have the liability waiver of the EUA product,” said Kirsch. “But once the Pfizer vaccine is fully approved in kids, then Pfizer gets liability waiver on all age groups due to a feature in federal law for child vaccines (NCVIA). At that time, they are done. They can market the COVID vaccine products under full approval for all age groups and face no liability when it kills or disables you.” (Related: More than 80% of children who received Pfizer’s covid injection suffered side effects.) The Centers for Disease Control and Prevention (CDC) said Pfizer is not planning to produce comirnaty while the vaccines with EUA remain available in the country. Comirnaty, according to the FDA, is a legally distinct product with with certain differences. Watch the video below to know more about the side effects on children of Pfizer’s COVID-19 vaccine. This video is from The Great Awakening channel on Brighteon.com. Follow Immunization.news for more news and information related to COVID-19 vaccines. SOURCES include: Dossier.Substack.com FreeWestMedia.com SteveKirsch.Substack.com Brighteon.comSteve Quayle: Truth about aliens and destruction of human race will be revealed
By Kevin Hughes // Share
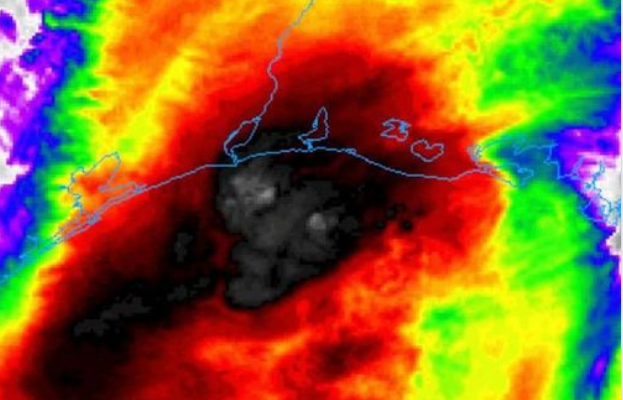
They are screwing with the weather maps: SUN is BAD!
By News Editors // Share
“Morally and intellectually corrupt”: UCLA professor resigns in protest over viewpoint intolerance
By News Editors // Share
‘Openly queer teacher’ admits to socially transitioning 3rd grade students
By News Editors // Share
Eating black sesame may slash heart disease risk
By newseditors // Share
Florida sky mystery: Toxic heavy metals and bioengineered particles found in air and food samples
By finnheartley // Share